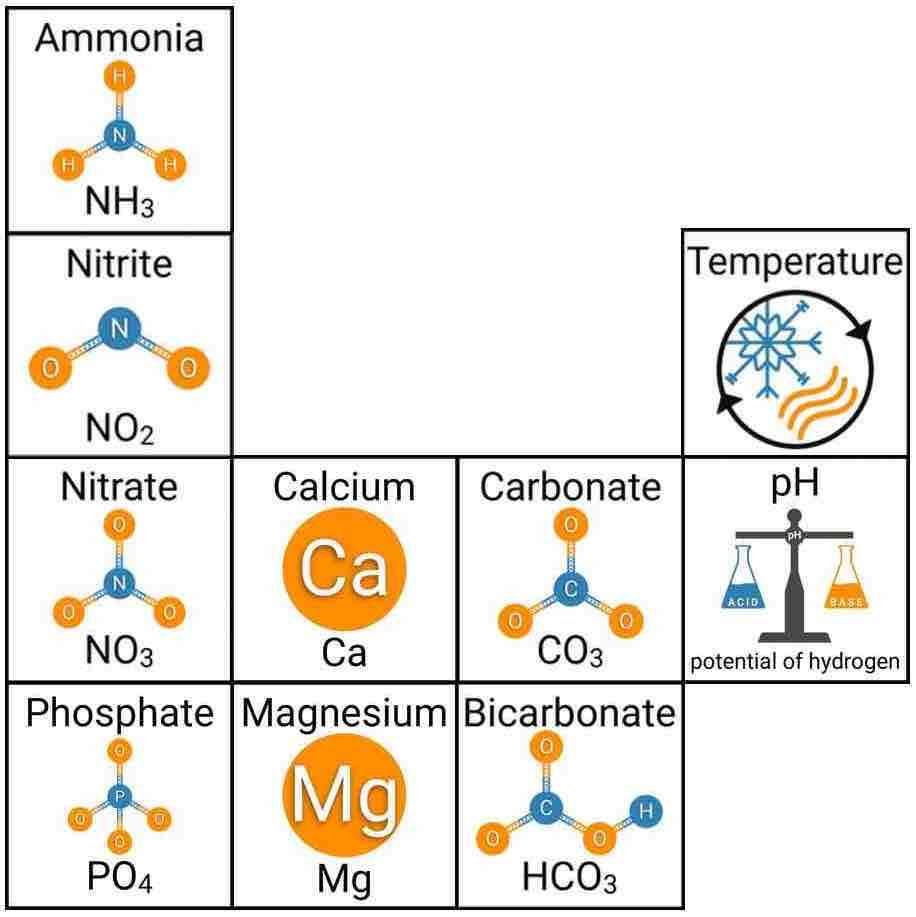
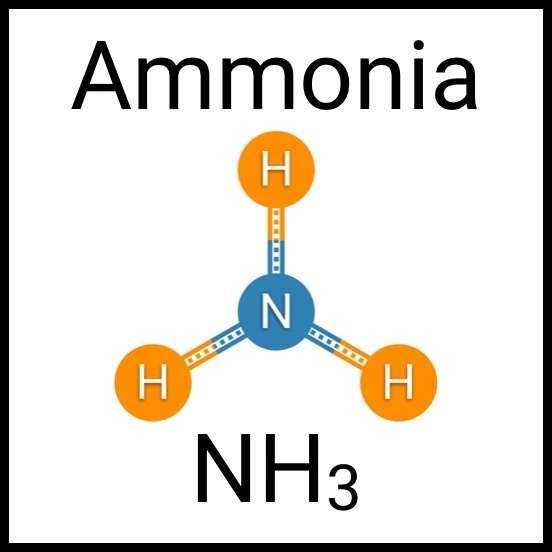
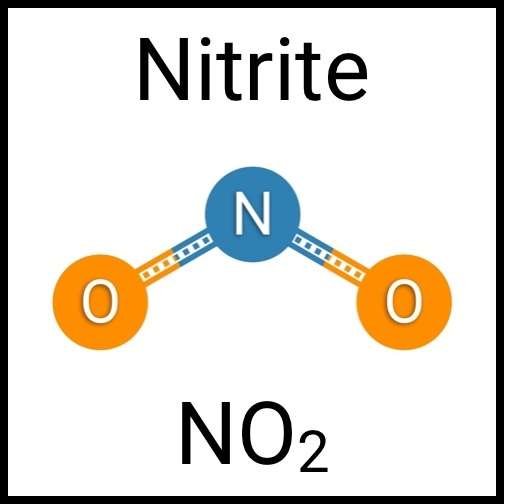
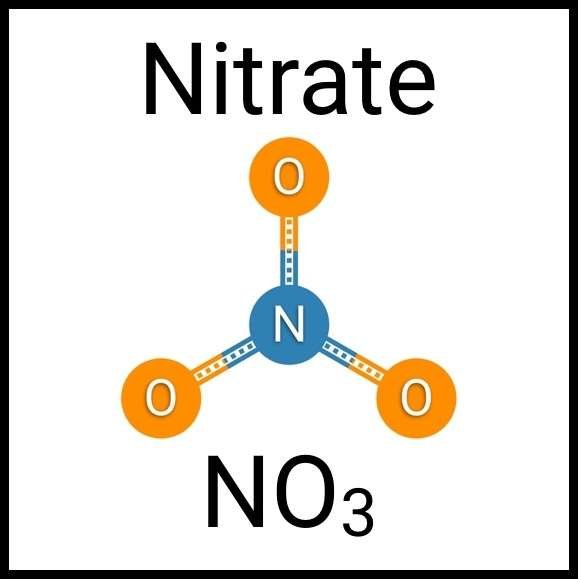
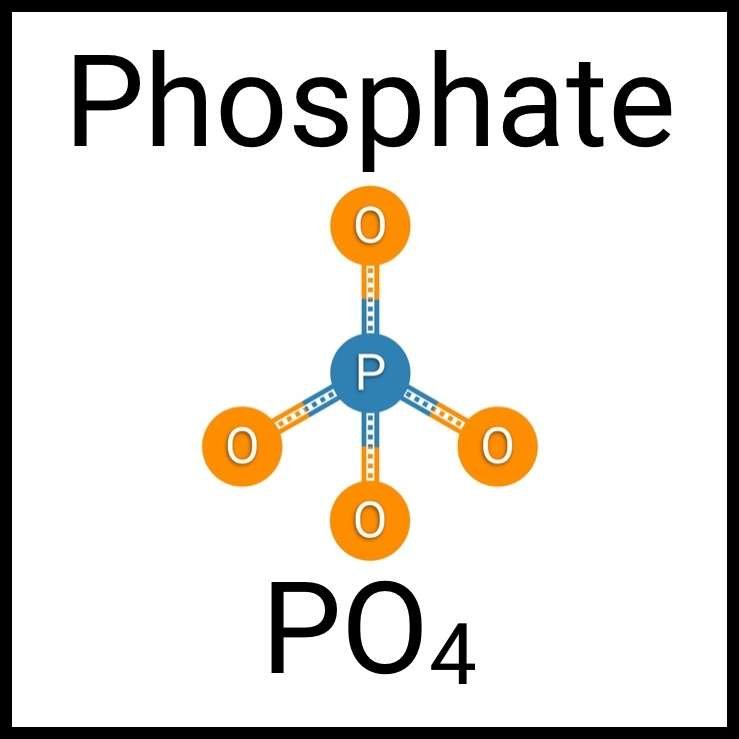
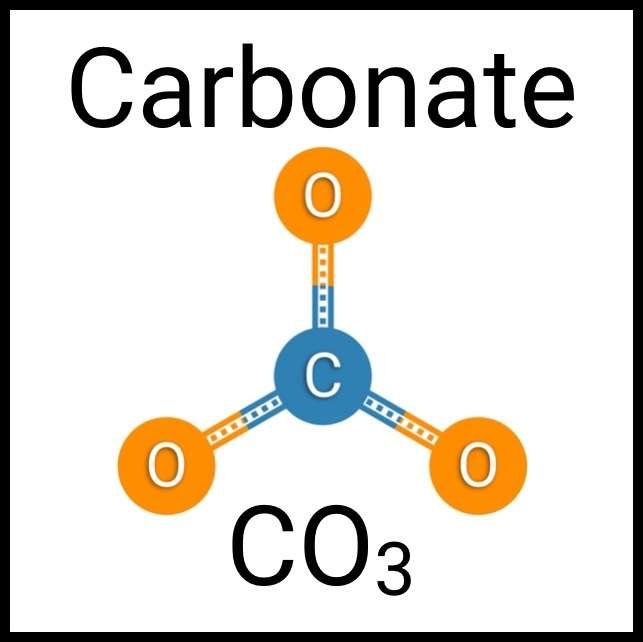
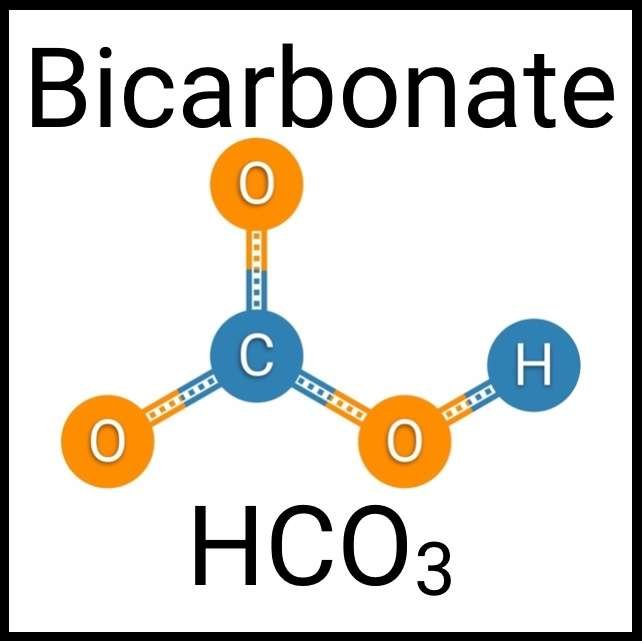
Every healthy ecosystem requires a balanced environment. Weekly water tests are invaluable to making sure your aquarium’s levels are in check. Understanding water chemistry and maintaining optimum levels of key nutrients, minerals and other indicators will protect aquatic life and allow the organisms in your care to thrive and radiate. Below are the key parameters to monitor for planted freshwater aquaria.